Chemistry class meets global catastrophe in real time.

Your morning coffee has more in common with the ocean than you might think. Both are becoming increasingly acidic, but while your coffee buzz wears off in a few hours, the ocean’s acid problem is permanent and accelerating. Scientists have been tracking this chemical transformation for decades, and the numbers are getting scary fast. We’re not talking about a distant environmental concern that future generations will handle. This is happening right now, measurably changing the fundamental chemistry of Earth’s largest ecosystem. The implications stretch far beyond dead coral reefs and into the basic food webs that keep billions of people fed.
1. Carbon dioxide transforms seawater into weak acid daily.
The ocean absorbs roughly 30% of all carbon dioxide we pump into the atmosphere, which sounds like a good thing until you understand the chemistry. When CO2 dissolves in seawater, it forms carbonic acid, the same stuff that gives soda its bite. According to the National Oceanic and Atmospheric Administration, ocean acidity has increased by 30% since the Industrial Revolution began, representing the fastest change in ocean chemistry in 300 million years.
Every day, the world’s oceans soak up about 22 million tons of carbon dioxide. That’s like dumping the equivalent of 150,000 cars worth of CO2 directly into the water. The ocean’s pH level has dropped from 8.2 to 8.1, which doesn’t sound dramatic until you remember that pH is measured on a logarithmic scale. This seemingly small change represents a massive shift in ocean chemistry that’s accelerating as we continue burning fossil fuels.
2. Shell-building creatures struggle with dissolving calcium carbonate structures.

Imagine trying to build a house while someone keeps dissolving your bricks faster than you can lay them. That’s exactly what’s happening to every creature in the ocean that builds shells or skeletons from calcium carbonate. More acidic water makes it exponentially harder for these animals to extract the materials they need from seawater, while simultaneously making their existing shells more vulnerable to dissolution. The process becomes a losing battle where energy spent on shell maintenance leaves less available for growth, reproduction, and survival, as reported by marine biologists studying Pacific oyster populations.
Oysters, mussels, clams, and sea snails are already showing measurably thinner shells in more acidic waters. But the problem extends far beyond these obvious cases. Microscopic plankton that form the base of ocean food webs also build calcium carbonate structures. When these tiny creatures struggle to survive, the effects ripple upward through every level of marine life.
3. Coral reefs bleach faster in increasingly acidic conditions.
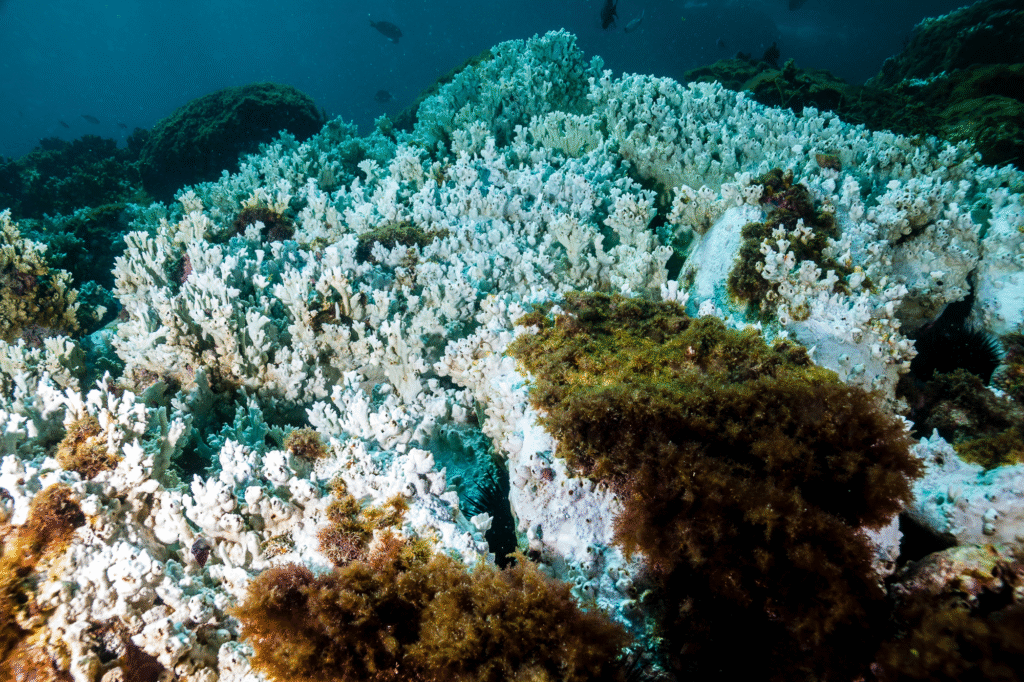
Coral reefs aren’t just pretty underwater gardens for vacation photos. They’re complex ecosystems that support roughly 25% of all marine species despite covering less than 1% of the ocean floor. Acidification attacks coral from two directions, weakening their calcium carbonate skeletons while making them more susceptible to bleaching events caused by warming waters. Research published in the journal Science shows that coral calcification rates have declined by 14% since 1990, with the steepest drops occurring in the most acidic waters, as discovered by Australian marine researchers tracking Great Barrier Reef health.
The relationship between ocean chemistry and coral health creates a devastating feedback loop. Stressed corals become more vulnerable to disease, pollution, and temperature changes. Once a reef system begins deteriorating, it loses its ability to buffer against further acidification, accelerating the decline. Entire reef ecosystems that took thousands of years to develop can collapse within decades under these combined pressures.
4. Plankton populations crash when water chemistry shifts dramatically.

Those microscopic floating creatures you’ve never thought about are literally holding up the entire ocean food web. Many plankton species build delicate shells from calcium carbonate, making them particularly vulnerable to acidification. When plankton populations crash, everything that eats them faces starvation, creating ripple effects that travel all the way up to large fish, seabirds, and marine mammals.
The timing couldn’t be worse. Plankton also play a crucial role in removing CO2 from the atmosphere through photosynthesis and by transporting carbon to deep ocean sediments when they die. Fewer plankton means less natural carbon removal, accelerating the very problem that’s killing them. This creates a vicious cycle where acidification reduces the ocean’s ability to help solve the climate crisis.
5. Fish lose their sense of smell in acid waters.

Fish navigate their underwater world largely through chemical signals, using their sense of smell to find food, avoid predators, and locate suitable habitat. Acidic water interferes with these chemical communications in ways scientists are just beginning to understand. Young fish become disoriented, swimming toward predators instead of away from them, while adults struggle to locate feeding grounds they’ve used for years.
The sensory disruption affects species differently, but the overall pattern is clear. Fish communities in more acidic waters show altered behavior patterns, reduced survival rates, and disrupted reproduction cycles. Commercial fish populations that depend on precise navigation for spawning migrations face particular challenges as ocean chemistry continues changing faster than species can adapt.
6. Shellfish farming industries face unprecedented production challenges.

Pacific Northwest oyster farms are already dealing with acidification in real time. Hatcheries have installed sophisticated monitoring systems and buffer tanks to protect baby oysters during their most vulnerable early development stages. Production costs are skyrocketing as farmers implement expensive workarounds for problems that didn’t exist a generation ago.
The industry represents a canary in the coal mine for global seafood production. What’s happening to oyster farms today previews the challenges facing all marine aquaculture as acidification spreads. Farmers are racing to develop acid-resistant species and protective technologies, but these solutions remain expensive and limited in scope compared to the scale of the problem.
7. Deep ocean currents carry acid waters across continents.

Ocean acidification isn’t staying put in areas with the highest CO2 emissions. Deep water currents transport chemically altered water around the globe, spreading acidification to remote areas that seem far removed from industrial activity. Antarctic waters are showing rapid acidification despite being thousands of miles from major pollution sources.
The global circulation system means that even dramatic emissions reductions in one region won’t immediately protect that area’s marine ecosystems. Acid waters formed decades ago in distant oceans are just now reaching some coastal areas. This time lag makes acidification particularly insidious because the full effects of today’s emissions won’t be felt for years or decades.
8. Arctic waters acidify faster than anywhere else on Earth.

Cold water holds more CO2 than warm water, making polar regions especially vulnerable to acidification. Arctic Ocean pH levels are dropping twice as fast as the global average, threatening ecosystems that are already stressed by rapid warming and ice loss. The combination of acidification and temperature changes creates compound threats that Arctic species have never faced in their evolutionary history.
Indigenous communities that depend on traditional marine resources are witnessing changes that elders say have no precedent in oral histories. Species that have supported Arctic cultures for thousands of years are experiencing population crashes and behavioral changes that threaten both ecological stability and food security for remote communities.
9. Coastal waters concentrate acid conditions near population centers.

Rivers and coastal runoff carry additional nutrients and pollutants that can amplify acidification effects in nearshore waters. These areas often support the most productive marine ecosystems and the largest human populations dependent on ocean resources. Coastal upwelling brings naturally more acidic deep water to the surface, creating zones where pH levels are already approaching critical thresholds for many species.
The concentration of acidification in coastal areas means that billions of people who depend on nearshore fisheries and marine ecosystems will experience the most severe impacts first. Small island nations and coastal communities in developing countries face particular challenges because they often lack resources to adapt fishing practices or develop alternative food sources.
10. Recovery timelines stretch beyond human civilization spans.

Even if we stopped all CO2 emissions tomorrow, ocean acidification would continue for decades as the atmosphere and oceans reach chemical equilibrium. The ocean’s natural buffering systems are already overwhelmed, and restoring pre-industrial pH levels would take thousands of years. This permanence distinguishes acidification from other environmental problems that might be reversible within human timescales.
The geological record shows that previous acidification events triggered mass extinction events that reset marine ecosystems completely. Recovery involved the evolution of entirely new species adapted to different ocean chemistry. For current marine life, including species that humans depend on for food and economic activity, there may be no adaptation period. The changes are happening too fast for evolutionary responses to keep pace.
